Accelerate Your MedTech Innovations
TAG3 is your trusted partner, delivering comprehensive, turnkey solutions for medical device design, development and commercialization.
Your Comprehensive ISO
13485 Medtech Partner
Transform your ideas into innovative, functional medical devices using our expert team.
- Concept Generation
- Clinical Need Assessment and Definition
- Clinical System FMEA
- Product Concept Design
- IP Assessment / Development
- Preliminary Hazard Analysis
- Rapid Prototyping (including 3D printing)
- 3D Modeling
- In-house Machining
- Finite Element Analysis
- Mechanical Design
- Electrical Design
- Concept Verification Testing
- Requirements Development
- Risk Management Plan
From concept to prototype to manufacturable product, we streamline regulated and compliant development for faster, efficient solutions.
- Detailed Product Design
- Preliminary Process Development and Documentation
- Design and Process FMEA
- COGS Estimates and Review
- Component and Assembly Specifications
- Packaging and Labeling Specifications
- Design Control System
- Design Transfer Planning
- ISO 13485 Certified
- FDA Registered and Audited
- Development Plan
- Regulatory Strategy and Filings
- Supply Chain Development
- Clinical Builds
- Simulated Use Model / Testing
- Human Factor Engineering and Formative Testing
- Feasibility Studies
- Functional Testing
- Test Method Development
- Accelerated Age Testing
- Packaging Design and Development
- Design for Manufacturability
- 3D Modeling and Design
Scale your production with our ISO 13485 and GMP-compliant manufacturing processes.
- Catheter Development, Extrusion, Braiding, Coil Winding, Laminating & Coating
- Balloon Development
- Assembly
- Packaging
- Process Validations (IQ, OQ, and PQ)
- Design, Verification and Validation Testing
- Transfer to Manufacturing
- At TAG3 or into your Facility
- Manufacturing Process and Test Method Verifications / Validations
- Manufacturing Tooling
- Regulatory Filings
- Commercial Readiness Planning
- Commercial Production
Develop
From concept to prototype to manufacturable product, we streamline regulated and compliant development for faster, efficient solutions.
Manufacture
Scale your production with our ISO 13485 and GMP-compliant manufacturing processes and facility.
From early ideation and prototyping to ISO 13485 compliant product development, regulatory submission, and manufacturing, we offer an integrated and seamless path from concept to commercialization.
Partner with us to drive the future of MedTech innovation and bring your revolutionary products to market faster than ever before.
TAG3 Partner Spotlight
Driving MedTech Success: How TAG3 Helped EvoEndo®, Inc. Innovate and Commercialize
Hear EvoEndo’s story of partnering with TAG3 Engineering to accelerate the development and commercialization of their innovative Single-Use Pediatric Unsedated Endoscopy System.
EvoEndo’s Story3.5mm (1.38″) Steerable Catheter with HD Endoscope and 2.0mm (0.078″) Working Channel
TAG3 Partner Spotlight
Driving MedTech Success: How TAG3 Helped PLU Ophthalmic™ Innovate and Commercialize AQUALUMEN™
Learn about PLU Ophthalmic’s story of partnering with TAG3 Engineering to accelerate the development and commercialization of AQUALUMEN™, their single use implant-free, precision-engineered surgical device for minimally invasive filtration surgeries for the treatment of ophthalmic disease or conditions.
PLU Ophthalmic’s Story“TAG3 identified design issues with our initial prototype and provided a plan to improve the device, leading to significant progress from phase zero to commercialization. The collaboration with TAG3 was incredibly fast, with a finished Class 1 device completed within 9 months. TAG3 is our commercial manufacturer and is responsible for manufacturing, logistics, packaging and sterilization.”
Alvaro Luque
Chief Executive Officer
TAG3 Partner Spotlight
Driving MedTech Success: How TAG3 served as a value-added strategic partner to Thrombolex™ throughout various stages of the product development process.
Learn more about Thrombolex’s story partnering with TAG3 Engineering.
Thrombolex’s Story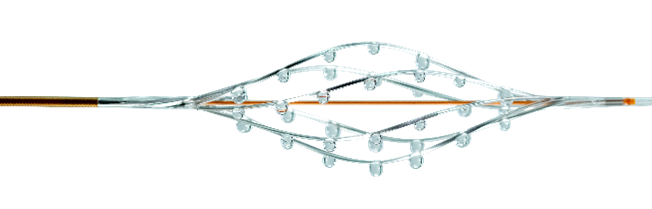
The BASHIR™ Endovascular Catheter uses a unique combination of both mechanical and pharmacological therapy to resolve thrombus. Targeted thrombolytics are delivered from the expandable infusion basket enabling the immediate restoration of blood flow and optimal resolution of arterial and venous thrombus.
















